Abstract
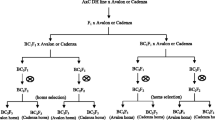
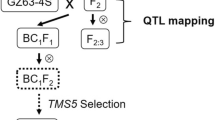
Near isogenic lines (NILs) are ideal material for a variety of genetic studies including validation of specific QTL. In the present study, eight pairs of NILs for grain weight were developed, seven in the background of Raj3765, and one in the background of K9107. For this purpose, marker-assisted selection (MAS) was used for the transfer of three grain weight QTL (QGw.ccsu-1A.2, QGw.ccsu-1A.3 and QGw.ccsu-1B.1) that were earlier identified in our laboratory. Two genotypes of each of the eight pairs of NILs, differed for QTL alleles (QTLHgw derived from the donor parent and the QTLLgw derived from the recipient parent). Each pair of NILs involved a solitary QTL except one NIL, which differed for all the three QTL. The difference in thousand grain weight (TGW) in two NILs of an individual pair ranged from 2.8 to 7.5 g, thus validating the effect of the QTL for TGW, although the quantum of difference did not always match the phenotypic variance of the corresponding QTL. As expected, the NILs which involved all the three QTL had the maximum difference of 7.5 g in TGW, and the NILs which involved QTL, QGw.ccsu-1A.2 had minimum average difference of 2.8 g for TGW. The NILs produced during the present study may be used in future for MAS and for fine mapping of TGW QTL.





Similar content being viewed by others
Abbreviations
- BC:
-
Back crossed
- CSAUAT:
-
Chandrashekhar Azad University of Agriculture and Technology
- DH:
-
Doubled haploid
- DTM:
-
Days to maturity
- HIPP:
-
Harvest index per plant
- MAS:
-
Marker-assisted selection
- NIL:
-
Near isogenic line
- PVE:
-
Phenotypic variance explained
- QTL:
-
Quantitative trait loci
- QTLHgw :
-
Donor parent high GW QTL allele
- QTLLgw :
-
Recipient parent low GW QTL allele
- RAU:
-
Rajasthan Agriculture University
References
Bai X, Wu B, Xing Y (2012) Yield-related QTLs and their applications in rice genetic improvement. J Integr Plant Biol 54:300–311
Breseghello F, Sorrells ME (2006a) Association mapping of kernel size and milling quality in wheat (Triticum aestivum L.) cultivars. Genetics 172:1165–1177
Breseghello F, Sorrells ME (2006b) Association analysis as a strategy for improvement of quantitative traits in plants. Crop Sci 46:1323–1330
Breseghello F, Sorrells ME (2007) QTL analysis of kernel size and shape in two hexaploid wheat mapping populations. Field Crops Res 101:172–179
Brinton J, Simmonds J, Minter F, Leverington-Waite M, Snape J, Uauy C (2017) Increased pericarp cell length underlies a major quantitative trait locus for grain weight in hexaploid wheat. New Phytol 215:1026–1038
Cabral AL, Jordan MC, Larson G, Somers DJ, Humphreys DG, McCartney CA (2018) Relationship between QTL for grain shape, grain weight, test weight, milling yield, and plant height in the spring wheat cross RL4452/‘AC Domain’. PLoS ONE 13:e0190681. https://doi.org/10.1371/journal.pone.0190681
Cosgrove DJ (2005) Growth of the plant cell wall. Nature Rev Mol Cell Biol 6:850–861
FAO (2017) Online statistical database: food balance. FAOSTAT. http://www.fao.org/faostat/en/. Accessed 27 Feb 2017
Farre A, Sayers L, Leverington-Waite M, Goram R, Orford S, Wingen L, Mumford C, Griffiths S (2016) Application of a library of near isogenic lines to understand context dependent expression of QTL for grain yield and adaptive traits in bread wheat. BMC Plant Biol 16:161
Gandhi SM, Sanghi AK, Nathawat KS, Bhatnagar MP (1963) Genotypic variability and correlation coefficient relating to grain yield and a few other quantitative characters in Indian wheats. Indian J Genet 24:1–8
Huang Y, Kong Z, Wu X, Cheng R, Yu D, Ma Z (2015) Characterization of three wheat grain weight QTLs that differentially affect kernel dimensions. Theor Appl Genet 128:2437–2445
Kuchel H, Williams KJ, Langridge P, Eagles HA, Jefferies SP (2007) Genetic dissection of grain yield in bread wheat: I. QTL analysis. Theor Appl Genet 115:1029–1041
Kumar N, Kulwal PL, Gaur A, Tyagi AK, Khurana JP, Khurana P, Balyan HS, Gupta PK (2006) QTL analysis for grain weight in common wheat. Euphytica 151:135–144
Kumar A, Mantovani EE, Seetan R, Soltani A, Echeverry-Solarte M, Jain S, Simsek S, Doehlert D, Alamri MS, Elias EM, Kianian SF, Mergoum M (2016) Dissection of genetic factors underlying wheat kernel shape and size in an elite × non adapted cross using a high density SNP linkage map. Plant Genome 9:1-22
Kumari S, Jaiswal V, Mishra VK, Paliwal R, Balyan HS, Gupta PK (2018) QTL mapping for some grain traits in bread wheat (Triticum aestivum L.). Physiol Mol Biol Plants 24(5):909–920
Lizana XC, Riegel R, Gomez LD, Herrera J, Isla A, Queen MM, Calderini DF (2010) Expansins expression is associated with grain size dynamics in wheat (Triticum aestivum L.). J Exp Bot 61:1147–1157
Ma J, Yan G, Liu CJ (2012) Development of near-isogenic lines for a major QTL on 3BL conferring Fusarium crown rot resistance in hexaploid wheat. Euphytica 183:147–152
Mir RR, Kumar N, Jaiswal V, Girdharwal N, Prasad M, Balyan HS, Gupta PK (2012) Genetic dissection of grain weight in bread wheat through quantitative trait locus interval and association mapping. Mol Breed 29:963–972
Muñoz M, Calderini DF (2015) Volume, water content, epidermal cell area, and XTH5 expression in growing grains of wheat across ploidy levels. Field Crops Res 173:30–40
Neumann K, Kobiljski B, Dencˇić S, Varshney RK, Börner A (2011) Genome-wide association mapping—a case study in bread wheat (Triticum aestivum L.). Mol Breed 27:37–58
Prasad M, Kumar N, Kulwal PL, Röder MS, Balyan HS, Dhaliwal HS, Gupta PK (2003) QTL analysis for grain protein content using SSR markers and validation studies using NILs in bread wheat. Theor Appl Genet 106:659–667
Radchuk V, Weier D, Radchuk R, Weschke W, Weber H (2011) Development of maternal seed tissue in barley is mediated by regulated cell expansion and cell disintegration and coordinated with endosperm growth. J Exp Bot 62:1217–1227
Ramya P, Chaubal A, Kulkarni K, Gupta L, Kadoo N, Dhaliwal HS, Chhuneja P, Lagu M, Gupta V (2010) QTL mapping of 1000-kernel weight, kernel length, and kernel width in bread wheat (Triticum aestivum L.). J Appl Genet 51:421–429
Reif JC, Gowda M, Maurer HP, Longin CFH, Korzun V, Ebmeyer E, Bothe R, Pietsch C, Wurschum T (2011) Association mapping for quality traits in soft winter wheat. Theor Appl Genet 122:961–970
Röder MS, Huang XQ, Börner A (2008) Fine mapping of the region on wheat chromosome 7D controlling grain weight. Funct Integr Genom 8:79–86
Sidwell RJ, Smith EL, McNew RW (1976) Inheritance and interrelationships of grain yield and selected yield-related traits in a hard red winter wheat cross. Crop Sci 16:650–654
Simmonds J, Scott P, Leverington-Waite M, Turner AS, Brinton J, Korzun V, Snape J, Uauy C (2014) Identification and independent validation of a stable yield and thousand grain weight QTL on chromosome 6A of hexaploid wheat (Triticum aestivum L.). BMC Plant Biol 14:191
Tanabata T, Shibaya T, Hori K, Ebana K, Yano M (2012) SmartGrain: high-throughput phenotyping software for measuring seed shape through image analysis. Plant Physiol 160:1871–1880
Tyagi S, Mir RR, Balyan HS, Gupta PK (2015) Interval mapping and meta-QTL analysis of grain traits in common wheat (Triticum aestivum L.). Euphytica 201:367–380
Wang RX, Hai L, Zhang XY, You GX, Yan CS, Xiao HS (2009) QTL mapping for grain filling rate and yield related trait in RILs of the Chinese winter population Heshangmai × Yu8679. Theor Appl Genet 118:313–325
Wang L, Cui F, Wang J, Jun L, Ding A, Zhao C, Li X, Feng D, Gao J, Wang H (2012) Conditional QTL mapping of protein content in wheat with respect to grain yield and its components. J Genet 91:303–312
Wang Y, Zhen S, Luo N, Han C, Lu X, Li X, Xia X, He Z, Yan Y (2016) Low molecular weight glutenin subunit gene Glu-B3h confers superior dough strength and bread making quality in wheat (Triticum aestivum L.). Sci Rep 6:27182. https://doi.org/10.1038/srep27182
Zhai H, Feng Z, Du X, Song Y, Liu X, Qi Z Song L, Li J, Li L, Peng H, Hu Z, Yao Y, Xin M, Xiao S, Sun Q, Ni Z (2018) A novel allele of TaGW2-A1 is located in a finely mapped QTL that increases grain weight but decreases grain number in wheat (Triticum aestivum L.). Theor Appl Genet 131:539–553
Zhang W, Li A, Tian J, Zhao L (2012) Development of near isogenic lines of wheat carrying different spike branching genes and their agronomic and spike characters. J Agric Sci 4:215–221
Zhang H, Chena J, Li R, Deng Z, Zhang K, Liu B, Tiana J (2016) Conditional QTL mapping of three yield components in common wheat (Triticum aestivum L.). Crop J 4:220–228
Zhou WC, Kolb FL, Bai GH, Domier LL, Boze LK, Smith NJ (2003) Validation of a major QTL for scab resistance with SSR markers and use of marker-assisted selection in wheat. Plant Breed 122:40–46
Zikhali M, Leverington-Waite M, Fish L, Simmonds J, Orford S, Wingen LU, Goram R, Gosman N, Bentley A, Griffiths S (2014) Validation of a 1DL earliness per se (eps) flowering QTL in bread wheat (Triticum aestivum L.). Mol Breed 34:1023–1033
Acknowledgements
The authors like to thank The Head, Department of Genetics and Plant Breeding, CCS University (Meerut, India) for providing facilities. PKG and HSB were each awarded the position of INSA Senior Scientist. PKG was also awarded a National Academy of Sciences India (NASI) Senior Scientist Platinum Jubilee Fellowship during the tenure of this research work. SK was awarded a JRF/SRF in a research project funded by the Department of Biotechnology, Government of India, New Delhi. Thanks are also due to Mr. Saripalli Gautam for his help in preparing the figures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kumari, S., Mir, R.R., Tyagi, S. et al. Validation of QTL for grain weight using MAS-derived pairs of NILs in bread wheat (Triticum aestivum L.). J. Plant Biochem. Biotechnol. 28, 336–344 (2019). https://doi.org/10.1007/s13562-018-0485-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13562-018-0485-3