Abstract
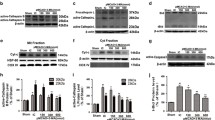
Hydrogen sulfide (H2S), an endogenous gaseous signal molecule, exhibits protective effect against ischemic injury. However, its underlying mechanism is not fully understood. We have recently reported that exogenous H2S decreases the accumulation of autophagic vacuoles in mouse brain with ischemia/reperfusion (I/R) injury. To further investigate whether this H2S-induced reduction of autophagic vacuoles is caused by the decreased autophagosome synthesis and/or the increased autophagic degradation inautophagic flux, we performed in vitro and in vivo studies using SH-SY5Y cells for the oxygen and glucose deprivation/reoxygenation (OGD/R) and mice for the cerebral I/R, respectively. NaHS (a donor of H2S) treatment significantly increased cell viability and reduced cerebral infarct volume. NaHS treatment reduced the OGD/R-induced elevation in LC3-II (an autophagic marker), which was completely reversed by co-treatment with an autophagic flux inhibitor bafilomycin A1 (BafA1). However, H2S did not affect the OGD/R-induced increase of the ULK1 self-association and decrease of the ATG13 phosphorylation, which are the critical steps for the initiation of autophagosome formation. Cerebral I/R injury caused an increase in LC3-II, a decrease in p62 and the accumulation of autophagosomes in the cortex and the hippocampus, which were inhibited by NaHS treatment. This H2S-induced decline of LC3-II in ischemic brain was reversed by BafA1. Moreover, BafA1 treatment abolished the protection of H2S on the cerebral infarction. Collectively, the neuroprotection of exogenous H2S against ischemia/hypoxia and reperfusion/reoxygenation injury is mediated by the enhancement of autophagic degradation.





Similar content being viewed by others
References
Calvert JW, Elston M, Nicholson CK, Gundewar S, Jha S, Elrod JW, Ramachandran A, Lefer DJ (2010) Genetic and pharmacologic hydrogen sulfide therapy attenuates ischemia-induced heart failure in mice. Circulation 122:11–19
Driessen S, Berleth N, Friesen O, Loffler AS, Bohler P, Hieke N, Stuhldreier F, Peter C, Schink KO, Schultz SW, Stenmark H, Holland P, Simonsen A, Wesselborg S, Stork B (2015) Deubiquitinase inhibition by WP1130 leads to ULK1 aggregation and blockade of autophagy. Autophagy 11:1458–1470
Fu Z, Liu X, Geng B, Fang L, Tang C (2008) Hydrogen sulfide protects rat lung from ischemia-reperfusion injury. Life Sci 82:1196–1202
Gheibi S, Aboutaleb N, Khaksari M, Kalalian-Moghaddam H, Vakili A, Asadi Y, Mehrjerdi FZ, Gheibi A (2014) Hydrogen sulfide protects the brain against ischemic reperfusion injury in a transient model of focal cerebral ischemia. J Mol Neurosci 54:264–270
Han SJ, Kim JI, Park JW, Park KM (2015) Hydrogen sulfide accelerates the recovery of kidney tubules after renal ischemia/reperfusion injury. Nephrol Dial Transplant 30:1497–1506
Hu H, Shi Y, Chen Q, Yang W, Zhou H, Chen L, Tang Y, Zheng Y (2008) Endogenous hydrogen sulfide is involved in regulation of respiration in medullary slice of neonatal rats. Neuroscience 156:1074–1082
Hu LF, Lu M, Hon Wong PT, Bian JS (2011) Hydrogen sulfide: neurophysiology and neuropathology. Antioxid Redox Signal 15:405–419
Jackman K, Kunz A, Iadecola C (2011) Modeling focal cerebral ischemia in vivo. Methods Mol Biol 793:195–209
Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, Adeli K, Agholme L, Agnello M, Agostinis P, Aguirre-Ghiso JA et al (2012) Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 8:445–544
Mizushima N, Komatsu M (2011) Autophagy: renovation of cells and tissues. Cell 147:728–741
Nazio F, Strappazzon F, Antonioli M, Bielli P, Cianfanelli V, Bordi M, Gretzmeier C, Dengjel J, Piacentini M, Fimia GM, Cecconi F (2013) mTOR inhibits autophagy by controlling ULK1 ubiquitylation, self-association and function through AMBRA1 and TRAF6. Nat Cell Biol 15:406–416
Papadakis M, Hadley G, Xilouri M, Hoyte LC, Nagel S, McMenamin MM, Tsaknakis G, Watt SM, Drakesmith CW, Chen R, Wood MJ, Zhao Z, Kessler B, Vekrellis K, Buchan AM (2013) Tsc1 (hamartin) confers neuroprotection against ischemia by inducing autophagy. Nat Med 19:351–357
Pearson RJ, Wilson T, Wang R (2006) Endogenous hydrogen sulfide and the cardiovascular system-what's the smell all about? Clin Invest Med 29:146–150
Perez-Rodriguez D, Anuncibay-Soto B, Llorente IL, Perez-Garcia CC, Fernandez-Lopez A (2015) Hippocampus and cerebral cortex present a different autophagic response after oxygen and glucose deprivation in an ex vivo rat brain slice model. Neuropathol Appl Neurobiol 41:e68–e79
Qin AP, Liu CF, Qin YY, Hong LZ, Xu M, Yang L, Liu J, Qin ZH, Zhang HL (2010) Autophagy was activated in injured astrocytes and mildly decreased cell survival following glucose and oxygen deprivation and focal cerebral ischemia. Autophagy 6:738–753
Qu K, Chen CP, Halliwell B, Moore PK, Wong PT (2006) Hydrogen sulfide is a mediator of cerebral ischemic damage. Stroke 37:889–893
Ren C, Du A, Li D, Sui J, Mayhan WG, Zhao H (2010) Dynamic change of hydrogen sulfide during global cerebral ischemia-reperfusion and its effect in rats. Brain Res 1345:197–205
Sagias FG, Mitry RR, Hughes RD, Lehec SC, Patel AG, Rela M, Mieli-Vergani G, Heaton ND, Dhawan A (2010) N-acetylcysteine improves the viability of human hepatocytes isolated from severely steatotic donor liver tissue. Cell Transplant 19:1487–1492
Shui M, Liu X, Zhu Y, Wang Y (2016) Exogenous hydrogen sulfide attenuates cerebral ischemiareperfusion injury by inhibiting autophagy in mice. Can J Physiol Pharmacol 22:1–6
Thal SC, Thal SE, Plesnila N (2010) Characterization of a 3-vessel occlusion model for the induction of complete global cerebral ischemia in mice. J Neurosci Methods 192:219–227
Vandiver MS, Snyder SH (2012) Hydrogen sulfide: a gasotransmitter of clinical relevance. J Mol Med-Jmm 90:255–263
Wang W, Fang H, Groom L, Cheng A, Zhang W, Liu J, Wang X, Li K, Han P, Zheng M, Yin J, Mattson MP, Kao JP, Lakatta EG, Sheu SS, Ouyang K, Chen J, Dirksen RT, Cheng H (2008) Superoxide flashes in single mitochondria. Cell 134:279–290
Wang D, Ma Y, Li Z, Kang K, Sun X, Pan S, Wang J, Pan H, Liu L, Liang D, Jiang H (2012a) The role of AKT1 and autophagy in the protective effect of hydrogen sulphide against hepatic ischemia/reperfusion injury in mice. Autophagy 8:954–962
Wang P, Guan YF, Du H, Zhai QW, Su DF, Miao CY (2012b) Induction of autophagy contributes to the neuroprotection of nicotinamide phosphoribosyltransferase in cerebral ischemia. Autophagy 8:77–87
Wang Y, Jia J, Ao G, Hu L, Liu H, Xiao Y, Du H, Alkayed NJ, Liu CF, Cheng J (2014) Hydrogen sulfide protects blood-brain barrier integrity following cerebral ischemia. J Neurochem 129:827–838
Xu F, Gu JH, Qin ZH (2012) Neuronal autophagy in cerebral ischemia. Neurosci Bull 28:658–666
Zhang XJ, Chen S, Huang KX, Le WD (2013) Why should autophagic flux be assessed? Acta Pharmacol Sin 34:595–599
Zhu Y, Bu Q, Liu X, Hu W, Wang Y (2014) Neuroprotective effect of TAT-14-3-3epsilon fusion protein against cerebral ischemia/reperfusion injury in rats. PLoS One 9:e93334
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81302763, 81302816, 81573333 and 81503060).
Author information
Authors and Affiliations
Contributions
Participated in research design: Zhu, Shui, Hu, Wang; Conducted experiments: Zhu, Shui; Performed data analysis: Zhu, Shui, Liu; Wrote or contributed to the writing of the manuscript: Zhu, Hu, Wang. All authors have read, revised and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors state that they have no competing interests.
Rights and permissions
About this article
Cite this article
Zhu, Y., Shui, M., Liu, X. et al. Increased autophagic degradation contributes to the neuroprotection of hydrogen sulfide against cerebral ischemia/reperfusion injury. Metab Brain Dis 32, 1449–1458 (2017). https://doi.org/10.1007/s11011-017-0014-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-017-0014-4