Abstract
Rationale
Variations in the effects of antidepressant drugs between different mouse strains are important for drug discovery and could lead to the identification of genes that predict differences in drug efficacy.
Objectives
This study compared behavioral baselines and dose-dependent responses to the selective serotonin reuptake inhibitor (SSRI) citalopram in eight inbred mouse strains (C57BL/6J, DBA/2J, C3H/HeJ, BALB/cJ, A/J, 129/SvEmsJ, 129/SvImJ, and BTBR) using the tail suspension test (TST).
Results
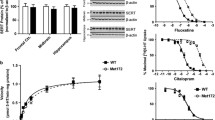
The DBA/2J, BALB/cJ, and BTBR strains were the most responsive to the effects of citalopram. Citalopram was least effective in the C57BL/6J and A/J strains. The antidepressant-like effects of citalopram in the TST were not correlated with changes in locomotor activity or deprivation-induced feeding behavior across the individual mouse strains, suggesting that patterns of sensitivity to citalopram are behaviorally specific and unlikely to result from pharmacokinetic variables. As an initial search for genetic polymorphisms causing differences in citalopram sensitivity, polymorphic forms of the tryptophan hydroxylase 2 (tph2) gene were genotyped and found to be not correlated with citalopram responsive (DBA/2J and BALB/cJ) and nonresponsive (A/J and C57BL/6J) strains.
Conclusions
The TST strain survey described here: (1) suggested the most appropriate strains for screening potential antidepressants, (2) identified parental strains appropriate for quantitative trait loci mapping of genomic loci regulating SSRI sensitivity, and (3) indicated appropriate background strains for measuring an antidepressant-like response to the SSRI citalopram. The pattern of response agrees with a previous mouse strain survey that examined sensitivity to fluoxetine in the forced swim test (Lucki I, Dalvi A, Mayorga AJ (2001) Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology 155:315–322).





Similar content being viewed by others
References
Arias B, Catalan R, Gasto C, Imaz ML, Gutierrez B, Pintor L et al (2001) Genetic variability in the promoter region of the serotonin transporter gene is associated with clinical remission of major depression after long-term treatment with citalopram. World J Biol Psychiatry 2:9S
Brocco M, Dekeyne A, Veiga S, Girardon S, Millan MJ (2002) Induction of hyperlocomotion in mice exposed to a novel environment by inhibition of serotonin reuptake. A pharmacological characterization of diverse classes of antidepressant agents. Pharmacol Biochem Behav 71:667–680
Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R (2003) Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 301:386–389
Crowley JJ, Lucki I (2005) Opportunities to discover genes regulating depression and antidepressant response from rodent behavioral genetics. Curr Pharm Des 11:157–169
Crowley JJ, Jones MD, O'Leary OF, Lucki I (2004) Automated tests for measuring the effects of antidepressants in mice. Pharmacol Biochem Behav 78:269–274
Cryan JF, Mombereau C, Vassout A (2005) The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev in press
David DJP, Renard CE, Jolliet P, Hascoet M, Bourin M (2003) Antidepressant-like effects in various mice strains in the forced swimming test. Psychopharmacology 166:373–382
Durham LK, Webb SM, Milos PM, Clary CM, Seymour AB (2004) The serotonin transporter polymorphism, 5HTTLPR, is associated with a faster response time to sertraline in an elderly population with major depressive disorder. Psychopharmacology 174:525–529
Edenberg HJ, Foroud T, Conneally PM, Sorbel JJ, Carr K, Crose C et al (1997) Initial genomic scan of the NIMH genetics initiative bipolar pedigrees: chromosomes 3, 5, 15, 16, 17, and 22. Am J Med Genet 74:238–246
Hyttel J (1993) Comparative pharmacology of selective serotonin reuptake inhibitors. Nord J Psychiatry 47(Suppl 30):5–20
Kim DK, Lim SW, Lee S, Sohn SE, Kim S, Hahn CG et al (2000) Serotonin transporter gene polymorphism and antidepressant response. Neuroreport 11:215–219
Kremer I, Bachner-Melman R, Reshef A, Broude L, Nemanov L, Gritsenko I, Heresco-Levy U, Elizur Y, Ebstein RP (2005) Association of the serotonin transporter gene with smoking behavior. Am J Psychiatry 162:924–930
Lander ES, Botstein D (1989) Mapping mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics 121:185–199
Liu X, Gershenfeld HK (2001) Genetic differences in the tail-suspension test and its relationship to imipramine response among 11 inbred strains of mice. Biol Psychiatry 49:575–581
Lucki I, Dalvi A, Mayorga AJ (2001) Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology 155:315–322
Mayorga AJ, Lucki I (2001) Limitations on the use of the C57BL/6 mouse in the tail suspension test. Psychopharmacology 155:110–112
Nelson JC (1999) A review of the efficacy of serotonergic and noradrenergic reuptake inhibitors for treatment of major depression. Biol Psychiatry 46:1301–1308
Perrault GH, Morel E, Zivkovic B, Sanger DJ (1992) Activity of litoxetine and other serotonin uptake inhibitors in the tail suspension test in mice. Pharmacol Biochem Behav 42:45–47
Pollack BG, Ferrell RE, Mulsant BH, Mazumdar S, Miller M, Sweet RA et al (2000) Allelic variation in the serotonin transporter promoter affects onset of paroxetine treatment response in late-life depression. Neuropsychopharmacology 23:587–590
Ripoll N, David DJ, Dailly E, Hascoet M, Bourin M (2003) Antidepressant-like effects in various mice strains in the tail suspension test. Behav Brain Res 3633:1–8
Schinka JA, Busch RM, Robichaux-Keene N (2004) A meta-analysis of the association between the serotonin transporter gene polymorphism (5-HTTLPR) and trait anxiety. Mol Psychiatry 9:197–202
Serretti A, Lilli R, Smeraldi E (2002) Pharmacogenetics in affective disorders. Eur J Pharmacol 438:117–128
Shanks N, Anisman H (1989) Strain-specific effects of antidepressants on escape deficits induced by inescapable shock. Psychopharmacology 99:122–128
Smeraldi E, Zanardi R, Benedetti F, Di Bella D, Perez J, Catalano M (1998) Polymorphism within the promoter of the serotonin transporter gene and antidepressant efficacy of fluvoxamine. Mol Psychiatry 3:508–511
Steru L, Chermat R, Thierry B, Simon P (1985) The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology 85:367–370
Wehner JM, Radcliffe RA, Bowers BJ (2001) Quantitative genetics and mouse behavior. Annu Rev Neurosci 24:845–867
Yamada K, Watanabe A, Iwayama-Shigeno Y, Yoshikawa T (2003) Evidence of association between gamma-aminobutyric acid type A receptor genes located on 5q34 and female patients with mood disorders. Neurosci Lett 349:9–12
Yoshikawa T, Watanabe A, Ishitsuka Y, Nakaya A, Nakatani N (2002) Identification of multiple genetic loci linked to the propensity for “behavioral despair” in mice. Genome Res 12:357–366
Zanardi R, Benedetti F, Di Bella D, Catalano M, Smeraldi E (2000) Efficacy of paroxetine in depression is influenced by a functional polymorphism within the promoter of the serotonin transporter gene. J Clin Psychopharmacol 20:105–107
Zanardi R, Serretti A, Rossini D, Franchini L, Cusin C, Lattuada E et al (2001) Factors affecting fluvoxamine antidepressant activity: influence of pindolol and 5-HTTLPR in delusional and nondelusional depression. Biol Psychiatry 50:323–330
Zhang X, Beaulieu JM, Sotnikova TD, Gainetdinov RR, Caron MG (2004) Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science 305:217
Acknowledgements
The authors would like to thank Dr. Wade Berrettini for guidance with this project. This research was supported by NIH grants MH14654 and MH48152.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Crowley, J.J., Blendy, J.A. & Lucki, I. Strain-dependent antidepressant-like effects of citalopram in the mouse tail suspension test. Psychopharmacology 183, 257–264 (2005). https://doi.org/10.1007/s00213-005-0166-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-005-0166-5