Abstract
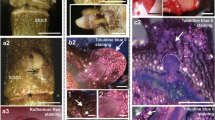
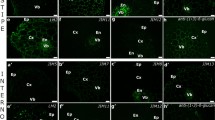
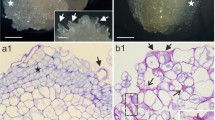
Arabinogalactan proteins (AGPs) are important proteoglycans regulating somatic embryogenesis in diverse plant species. Embryogenic cells of somatic embryos are covered by special extracellular cell wall layer called extracellular surface matrix network (ECMSN) at their early developmental stages. Here we show that highly embryogenic cell line AC78 of hybrid fir (Abies alba × Abies cephalonica) differs from very low-embryogenic cell line AC77 in the abundance, subcellular localization and deposition of subset of secreted AGPs. A specific AGP epitope containing Gal residues and reacting to Gal4 antibody is secreted and deposited into ECMSN, which covers the surface of the embryogenic cells showing high embryogenic and regeneration capacity in the cell line AC78. On the other hand, this Gal4 AGP epitope was not secreted and/or found on the surface of meristematic cells showing low embryogenic and regeneration capacity in the cell line AC77, as well as on the surface of non-embryogenic suspensor cells and callus cells in both cell lines AC77 and AC78. As a positive control, we have used another AGP epitope LM2 (containing glucuronic acid) showing no significant differences in these two Abies hybrid lines. This study defines specific AGPs containing β-(1→6)-galactotetraosyl group as a first molecular component of ECMSN covering embryogenic cells in gymnosperms.





Similar content being viewed by others
Abbreviations
- AC77:
-
Very low-embryogenic cell line of hybrid fir
- AC78:
-
Highly-embryogenic cell line of hybrid fir
- AGP:
-
Arabinogalactan protein
- Gal4 antibody:
-
Antibody raised against the β-(1→6)-galactotetraosyl group of arabinogalactan protein
- ECMSN:
-
Extracellular matrix surface network
- LM2 antibody:
-
Antibody recognizing arabinogalactan protein containing β-linked glucuronic acid residue
- SEM:
-
Scanning electron microscopy
References
Baluška F, Šamaj J, Wojtaszek P, Volkmann D, Menzel D (2003) Cytoskeleton-plasma membrane-cell wall continuum in plants. Emerging links revisited. Plant Physiol 133:482–491
Chapman A, Blervacq AS, Vasseur J, Hilbert JL (2000) Arabinogalactan-proteins in Cichorium somatic embryogenesis: effect of β-glucosyl Yariv reagent and epitope localization during embryo development. Planta 211:305–314
Chen T, Teng N, Wu X, Wang Y, Tang W, Šamaj J, Baluška F, Lin J (2007) Disruption of actin filaments by latrunculin B affects cell wall construction in Picea meyeri pollen tube by disturbing vesicle trafficking. Plant Cell Physiol 48:19–30
David A, Laine E, David H (1995) Somatic embryogenesis in Pinus caribaea. In: Jain SM, Gupta PK, Newton RJ (eds) Somatic embryogenesis in woody plants. Vol 3—Gymnosperms. Kluwer, Dordrecht, pp 145–181
Domon JM, Neutelings G, Roger D, David A, David H (2000) A basic chitinase-like protein secreted by embryogenic tissues of Pinus caribaea acts on arabinogalactan proteins extracted from the same cell lines. J Plant Physiol 2000:33–39
Dubois T, Dubois J, Guedira M, Diop A, Vasseur J (1992) SEM characterization of an extracellular matrix around somatic proembryos in roots of Cichorium. Ann Bot 70:119–124
Egertsdotter U, von Arnold S (1995) Importance of arabinogalactan proteins for the development of somatic embryos of Norway spruce (Picea abies). Physiol Plant 93:334–345
Filonova LH, Bozhkov PV, von Arnold S (2000) Developmental pathway of somatic embryogenesis in Picea abies as revealed by time-lapse tracking. J Exp Bot 51:249–264
Hurkman WJ, Tanaka CK (1986) Solubilisation of plant membrane proteins for analysis by two-dimensional gel electrophoresis. Plant Physiol 81:802–816
Jalonen P, von Arnold S (1991) Characterization of embryogenic cell lines of Picea abies in relation to their competence for maturation. Plant Cell Rep 10:383–387
Jasik J, Salajova T, Salaj J (1995) Developmental anatomy and ultrastructure or early somatic embryos of European black pine (Pinus nigra Arn.). Protoplasma 185:205–211
Jasik J, Salajova T, Kormutak A, Salaj J (1999) Somatic embryogenesis in hybrid firs. In: Jain SM (ed) Somatic embryogenesis in woody plants, vol 4. Kluwer, Dordrecht, pp 505–523
Kikuchi S, Ohinata A, Tsumuraya Y, Hashimoto Y, Kaneko Y, Matsushima H (1993) Production and characterisation of antibodies to the β-(1→6)-galactotetraosyl group and their interaction with arabinogalactan-proteins. Planta 190:525–535
Konieczny R, Swierczynska J, Czaplicki AZ,Bohdanowicz J (2007) Distribution of pectin and arabinogalactan protein epitopes during organogenesis from androgenic callus of wheat. Plant Cell Rep 26:355–363
Kreuger M, van Holst GJ (1993) Arabinogalactan proteins are essential in somatic embryogenesis of Daucus carota L. Planta 189:243–248
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
McCabe PF, Valentine TA, Forsberg LS, Pennell RI (1997) Soluble signals from cells identified at the cell wall establish a developmental pathway in carrot. Plant Cell 9:2225–2241
Mo LH, Egertsdotter U, von Arnold S (1996) Secretion of specific extracellular proteins by somatic embryos of Picea abies is dependent on embryo morphology. Ann Bot 77:143–152
Mogami N, Nakamura S, Nakamura N (1999) Immunolocalization of the cell wall components in Pinus densiflora pollen. Protoplasma 206:1–10
Namasivayam P, Skepper J, Hanke D (2006) Identification of a potential structural marker for embryogenic competency in the Brassica napus spp. oleifera embryogenic tissue. Plant Cell Rep 25:887–895
Nothnagel EA (1997) Proteoglycans and related components in plant cells. Int Rev Cytol 174:195–291
Saare-Surminski K, Preil W, Knox JP, Lieberei R (2000) Arabinogalactan proteins in embryogenic and non-embryogenic callus cultures of Euphorbia pulcherrima. Physiol Plant 108:180–187
Salajová T, Jasik J, Kormutak A, Salaj J, Hakman I (1996) Embryogenic culture initiation and somatic embryo development in hybrid firs (Abies alba × Abies cephalonica, and Abies alba × Abies numidica). Plant Cell Rep 15:527–530
Šamaj J, Bobák M, Blehová A, Krištín J, Auxtová-Šamajová O (1995) Developmental SEM observations of an extracellular matrix in embryogenic calli of Drosera rotundifolia and Zea mays. Protoplasma 186:45–49
Šamaj J, Baluška F, Volkmann D (1998) Cell- specific expression of two arabinogalactan-protein epitopes by monoclonal antibodies JIM8 and JIM13 in maize roots. Protoplasma 204:1–12
Šamaj J, Baluška F, Bobák M, Volkmann D (1999a) Extracellular matrix surface network of embryogenic units of friable maize callus contains arabinogalactan-proteins recognized by monoclonal antibody JIM4. Plant Cell Rep 18:369–374
Šamaj J, Ensikat HJ, Baluška F, Knox JP, Barthlott W, Volkmann D (1999b) Immunogold localization of plant surface arabinogalactan-proteins using glycerol liquid substitution and scanning electron microscopy. J Microsc 193:150–157
Šamaj J, Braun M, Baluška F, Ensikat HJ, Tsumuraya Y, Volkmann D (1999c) Specific localization of arabinogalactan-protein epitopes at surface of maize root hairs. Plant Cell Physiol 40:874–883
Šamaj J, Šamajová O, Peters M, Baluška F, Lichtscheidl I, Knox JP, Volkmann D (2000) Immunolocalization of LM2 arabinogalactan-protein epitope associated with endomembranes of plant cells. Protoplasma 212:186–196
Šamaj J, Bobák M, Blehová A, Preťová A (2006) Importance of cytoskeleton and cell wall in somatic embryogenesis. In: Mujib A, Šamaj J (eds) Somatic embryogenesis in plants: new approaches. Springer, Heidelberg, pp 35–50
Sardar HS, Yang J, Showalter AM (2006) Molecular interactions of arabinogalactan-proteins (AGPs) with cortical microtubules and F-actin in Bright Yellow-2 (BY-2) tobacco cultured cells. Plant Physiol (in press)
Serpe MD, Nothnagel EA (1999) Arabinogalactan-proteins in the multiple domains of the plant cell surface. Adv Bot Res 30:207–289
Showalter AM (2001) Arabinogalactan-proteins: structure, expression and function. Cell Mol Life Sci 58:1399–1417
Smallwood M, Yates EA, Willats WGT, Martin H, Knox JP (1996) Immunochemical comparison of membrane-associated and secreted arabinogalactan-proteins in rice and carrot. Planta 198:452–459
Stacey NJ, Roberts K, Knox JP (1990) Patterns of expression of the JIM4 arabinogalactan-protein epitope in cell cultures and during somatic embryogenesis in Daucus carota L. Planta 180:285–292
Thompson HJM, Knox JP (1998) Stage-specific responses of embryogenic carrot cell suspension cultures to arabinogalactan protein-binding β-glucosyl Yariv reagent. Planta 205:32–38
Van Hengel AJ, Tadesse Z, Immerzeel P, Schols H, van Kammen A, de Vries SC (2001) N-acetylglucosamine and glucosamine-containing arabinogalactan proteins control somatic embryogenesis. Plant Physiol 125:1880–1890
Vitha S, Baluška F, Braun M, Šamaj J, Volkmann D, Barlow PW (2000) Comparison of cryofixation and aldehyde fixation for plant actin immunocytochemistry: aldehydes do not destroy F-actin. Histochem J 32:457–466
von Arnold S, Hakman I (1988) Regulation of somatic embryo development in Picea abies by abscisic acid (ABA). J Plant Physiol 132:164–169
Acknowledgments
This work was supported by the grant from the Slovak Grant Agency VEGA, Project Nr. 2/5022/25, and DAAD-SAV project Nr. 62223.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L. Peña.
Electronic supplementary material
Below is the link to the electronic supplementary material.
299_2007_429_MOESM1_ESM.jpg
Supplementary Figure 1. Developmental SEM observations revealed progressive accumulation of ECMSN on the surface of embryogenic cells in the cell line AC78. Bars represent 3 µm for a; and 10 µm for b ESM1 (JPEG 34.8 kb)
299_2007_429_MOESM2_ESM.jpg
Supplementary Figure 2. Non-embryogenic calli of the cell line AC78 (a) and AC77 (c) were used as positive controls and labelled with Gal4 antibody (b and d, respectively). Note, that this epitope was restricted to the cytoplasm but not secreted to the cell wall in callus cells of both lines. Bars represent 50 µm for a, c; and 20 µm for b and d ESM2 (JPEG 35.5 kb)
Rights and permissions
About this article
Cite this article
Šamaj, J., Salaj, T., Matúšová, R. et al. Arabinogalactan-protein epitope Gal4 is differentially regulated and localized in cell lines of hybrid fir (Abies alba × Abies cephalonica) with different embryogenic and regeneration potential. Plant Cell Rep 27, 221–229 (2008). https://doi.org/10.1007/s00299-007-0429-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-007-0429-1