Abstract
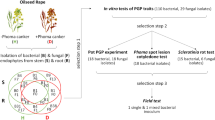
Recent and substantial yield losses of Styrian oil pumpkin (Cucurbita pepo L. subsp. pepo var. styriaca Greb.) are primarily caused by the ascomycetous fungus Didymella bryoniae but bacterial pathogens are frequently involved as well. The diversity of endophytic microbial communities from seeds (spermosphere), roots (endorhiza), flowers (anthosphere), and fruits (carposphere) of three different pumpkin cultivars was studied to develop a biocontrol strategy. A multiphasic approach combining molecular, microscopic, and cultivation techniques was applied to select a consortium of endophytes for biocontrol. Specific community structures for Pseudomonas and Bacillus, two important plant-associated genera, were found for each microenvironment by fingerprinting of 16S ribosomal RNA genes. All microenvironments were dominated by bacteria; fungi were less abundant. Of the 2,320 microbial isolates analyzed in dual culture assays, 165 (7%) were tested positively for in vitro antagonism against D. bryoniae. Out of these, 43 isolates inhibited the growth of bacterial pumpkin pathogens (Pectobacterium carotovorum, Pseudomonas viridiflava, Xanthomonas cucurbitae); here only bacteria were selected. Microenvironment-specific antagonists were found, and the spermosphere and anthosphere were revealed as underexplored reservoirs for antagonists. In the latter, a potential role of pollen grains as bacterial vectors between flowers was recognized. Six broad spectrum antagonists selected according to their activity, genotypic diversity, and occurrence were evaluated under greenhouse conditions. Disease severity on pumpkins of D. bryoniae was significantly reduced by Pseudomonas chlororaphis treatment and by a combined treatment of strains (Lysobacter gummosus, P. chlororaphis, Paenibacillus polymyxa, and Serratia plymuthica). This result provides a promising prospect to biologically control pumpkin diseases.





Similar content being viewed by others
References
Fruehwirth GO, Hermetter A (2007) Seeds and oil of the Styrian oil pumpkin: components and biological activities. Eur J Lipid Sci Tech 109:1128–1140
Dreikorn K (2002) The role of phytotherapy in treating lower urinary tract symptoms and benign prostatic hyperplasia. World J Urol 19:426–435
Huss H, Winkler J, Greimel C (2007) Der Pilz Didymella bryoniae schädigt steirischen Ölkürbisanbau: Fruchtfäule statt Kernöl. (The fungus Didymella bryoniae affect oil pumpkins in Styria: fruit rot instead of oil). Der Pflanzenarzt 60:14–16
Sitterly WR, Keinath AP (1996) Gummy stem blight. In: Zitter TA, Hopkins DL, Thomas CE (eds) Compendium of cucurbit diseases. American Phytological Society Press, St. Paul, pp 27–28
Keinath AP (2010) From native plants in central Europe to cultivated crops worldwide: the emergence of Didymella bryoniae as a cucurbit pathogen. Cucurbitaceae Conference, Charleston
Grube M, Fürnkranz M, Zitzenbacher S, Huss H, Berg G (2011) Emerging multi-pathogen disease caused by Didymella bryoniae and pathogenic bacteria on Styrian oil pumpkin. Europ J Plant Pathol. doi:10.1007/s10658-011-9829-8
Huss H (2011) Krankheiten und Schädlinge im Ölkürbisbau. (Diseases and pests in oil pumpkin). Der fortschrittliche Landwirt 3:30–33
Berg G, Smalla K (2009) Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microb Ecol 68:1–13
Compant S, Duffy B, Nowak J, Clement C, Barka EA (2005) Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71:4951–4959
Lugtenberg B, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Annu Rev Microbiol 63:541–556
Backman PA, Sikora RA (2008) Endophytes: an emerging tool for biological control. Biol Control 46:1–3
Sessitsch A, Coenye T, Sturz AV, Vandamme P, Barka EA, Salles JF, Van Elsas JD, Faure D, Reiter B, Glick BR, Wang-Pruski G, Nowak J (2005) Burkholderia phytofirmans sp. nov., a novel plant-associated bacterium with plant-beneficial properties. Int J Syst Evol Microbiol 55:1187–1192
Berg G, Hallmann J (2006) Control of plant pathogenic fungi with bacterial endophytes. In: Schulz B, Boyle C, Sieber T (eds) Microbial root endophytes. Springer, Berlin, pp 53–70
Berg G, Krechel A, Ditz M, Sikora RA, Ulrich A, Hallmann J (2005) Endophytic and ectophytic potato-associated bacterial communities differ in structure and antagonistic function against plant pathogenic fungi. FEMS Microb Ecol 51:215–229
Unge A, Jansson J (2001) Monitoring population size, activity, and distribution of gfp-luxAB-tagged Pseudomonas fluorescens SBW25 during colonization of wheat. Microb Ecol 41:290–300
Schwieger F, Tebbe CC (1998) A new approach to utilize PCR-single-strand-conformation polymorphism for 16S rRNA gene-based microbial community analysis. Appl Environ Microbiol 64:4870–4876
Schmid F, Grube M, Berg G (2011) Black fungi and associated bacterial communities in the phyllosphere of grapevine. Fungal Biol doi:10.1016/j.funbio.2011.04.004
Larena I, Salazar O, González V, Julián MC, Rubio V (1999) Design of a primer for ribosomal DNA internal transcribed spacer with enhanced specificity for ascomycetes. J Biotechnol 75:187–194
White TJ, Bruns TD, Lee S, Taylor J (1990) Analysis of phylogenetic relationship by amplification and direct sequencing of ribosomal RNA genes. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic, San Diego, pp 315–322
Bassam BJ, Caetano-Anolles G, Gresshoff PM (1991) Fast and sensitive silver staining of DNA in polyacrylamide gels. Anal Biochem 196:80–83
Altschul SF, Madden TL, Schaffer AA, Zhang JH, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research 25:3389–3402
Lo Piccolo S, Ferraro V, Alfonzo A, Settanni L, Ercolini D, Burruano S, Moschetti G (2010) Presence of endophytic bacteria in Vitis vinifera leaves as detected by fluorescence in situ hybridization. Ann Microbiol 60:161–167
Meier H, Amann R, Ludwig W, Schleifer KH (1999) Specific oligonucleotide probes for in situ detection of a major group of gram-positive bacteria with low DNA G+C content. Syst Appl Microbiol 22:186–196
Amann RI, Binder BJ, Olson RJ, Chisholm CW, Devereux R, Stahl DH (1990) Combination of 16S rRNA targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol 56:1919–1925
Rademaker JLW, De Bruijn FJ (1997) Characterization and classification of microbes by REP-PCR genomic fingerprinting and computer-assisted pattern analysis. In: Caetano-Anollés G, Gresshoff PM (eds) DNA markers: protocols, applications and overviews. Wiley, New York, pp 151–171
Costa R, Salles JF, Berg G, Smalla K (2006) Cultivation-independent analysis of Pseudomonas species in soil and in the rhizosphere of field-grown Verticillium dahliae host plants. Environ Microbiol 8:2136–2149
Junker RR, Loewel C, Gross R, Dötterl S, Keller A, Blüthgen N (2011) Composition of epiphytic bacterial communities differs on petals and leaves. Plant Biol doi: 10.1111/j.1438-8677.2011.00454.x
Dulla GF, Lindow SE (2009) Acyl-homoserine lactone-mediated cross talk among epiphytic bacteria modulates behavior of Pseudomonas syringae on leaves. ISME J 3:825–834
Berg G, Eberl L, Hartmann A (2005) The rhizosphere as a reservoir for opportunistic human pathogenic bacteria. Environ Microbiol 7:1673–1685
van Overbeek LS, Franke AC, Nijhuis EH, Groeneveld RM, da Rocha UN, Lotz LA (2011) Bacterial communities associated with Chenopodium album and Stellaria media seeds from arable soils. Microb Ecol. doi:10.1007/s00248-011-9845-4
Berg G, Roskot N, Steidle A, Eberl L, Zock A, Smalla K (2002) Plant-dependent genotypic and phenotypic diversity of antagonistic rhizobacteria isolated from different Verticillium host plants. Appl Environ Microbiol 68:3328–3338
Berg G, Opelt K, Zachow C, Lottmann J, Gotz M, Costa R, Smalla K (2006) The rhizosphere effect on bacteria antagonistic towards the pathogenic fungus Verticillium differs depending on plant species and site. FEMS Microb Ecol 56:250–261
Haas D, Défago G (2005) Biological control of soil-borne pathogens by fluorescent Pseudomonads. Nat Rev Microbiol 3:307–319
Müller H, Westendorf C, Leitner E, Chernin L, Riedel K, Schmidt S, Eberl L, Berg G (2009) Quorum-sensing effects in the antagonistic rhizosphere bacterium Serratia plymuthica HRO-C48. FEMS Microb Ecol 67:468–478
Postma J, Schilder MT, Bloem J, van Leeuwen-Haagsma WK (2008) Soil suppressiveness and functional diversity of the soil microflora in organic farming systems. Soil Biol Biochem 40:2394–2406
Danchin A, Sekowska A (2010) The role of information in evolutionary genomics of bacteria. In: Caetano-Anollés G (ed) Evolutionary genomics and systems biology. Wiley, New Jersey, pp 81–95
Acknowledgments
We thank Massimiliano Cardinale, Martina Köberl, and Christin Zachow (Graz) for valuable support. Furthermore, we want to thank Johanna Winkler (Saatzucht Gleisdorf) for excellent cooperation regarding pumpkin cultivars and field trials. The project was funded by the Austrian Federal Ministry of Agriculture, Forestry, Environment and Water Management and the governments of the Federal State of Styria.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fürnkranz, M., Lukesch, B., Müller, H. et al. Microbial Diversity Inside Pumpkins: Microhabitat-Specific Communities Display a High Antagonistic Potential Against Phytopathogens. Microb Ecol 63, 418–428 (2012). https://doi.org/10.1007/s00248-011-9942-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-011-9942-4