Abstract
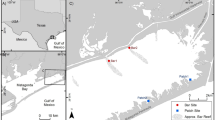
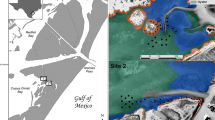
Oyster populations are often structured by both biotic interactions and abiotic stresses. Juvenile oysters, i.e., those most vulnerable to predation, face a wide range of predatory characteristics (size, mobility) such that predator identity might exert a strong influence on oyster populations. Likewise, oyster reef location, either as isolated patch reefs or saltmarsh-fringing reefs, can strongly influence the ecological processes impacting oyster populations. Therefore, this study sought to quantify the contribution of predator identity, in particular mesopredators, to oyster survival in the field, while also examining the role of landscape setting in predation. Using a multiple mesh size cage design, oyster survival was measured by excluding access to different groups of predators at both patch and fringing reef and reference sites in Hewlett’s Creek, Wilmington, North Carolina, in August/September 2013, while also monitoring settlement and recruitment at both reef locations from May to August 2013. The results indicated a significant cage by location interaction, indicating that the predator identity or modality was not the same across all sites. Despite this, at either reef location, oyster survival did not differ between 37-mm mesh cages, which allowed access only by mesopredators, and no-cage treatments, which allowed access to all predators, while survival was reduced by >20 % on fringing reefs relative to patch reefs. This study demonstrates the significant contribution of mesopredators, likely xanthid crabs, to oyster predation in the field at ambient predator densities and suggests that the differences in oyster abundance between patch and fringing reef locations are likely due to differential predation.





Similar content being viewed by others
References
Artabane S (2006) The effects of proximity to a subtidal channel on habitat utilization of intertidal oyster reefs. MS Thesis, University of North Carolina Wilmington
Bahr L, Lanier W (1981) The ecology of intertidal oyster reefs of the south Atlantic coast: a community profile. US Fish and Wildlife Service, Washington
Beck M, Brumbaugh R, Airoldi L, Carranza A, Coen L, Crawford C, Defeo O, Edgar G, Hancock B, Kay M, Lenihan H, Luckenbach M, Toropova C, Zhang G, Guo X (2011) Oyster reefs at risk and recommendations for conservation, restoration, and management. Bioscience 61:107–116
Carroll JM, Finelli CM (2014) Impacts of the ectoparasitic snail Boonea impressa on growth of postset juvenile oysters. J Mollusc Stud. doi:10.1093/mollus/eyu070
Carroll JM, Furman BT, Tettelbach ST, Peterson BJ (2012) Balancing the edge effects budget: bay scallop settlement and loss along a seagrass edge. Ecology 93:1637–1647
Coen L, Luckenbach M (2000) Developing success criteria goals for evaluating shellfish habitat restoration: ecological function or resource exploitation. Ecol Engin 15:323–343
Coen LD, Brumbaugh RD, Bushek D, Grizzle R, Luckenbach MW, Posey MH, Powers SP, Tolley SG (2007) Ecosystem services related to oyster restoration. Mar Ecol Prog Ser 341:303–307
Ebling F, Kitching J, Muntz L, Taylor C (1964) The ecology of Lough Ine. XIII. Experimental observations of the destruction of Mytilus edulis and Nucella lapillus by crabs. J Animal Ecol 33:73–82
Eggleston DB (1990) Behavioral mechanisms underlying variable functional responses of blue crabs, Callinectes sapidus feeding on juvenile oysters, Crassostrea virginica. J Animal Ecol 59:615–630
Eggleston D, Lipcius R, Hines A (1992) Density-dependent predation by blue crabs upon infaunal clam species with contrasting distribution and abundance patterns. Mar Ecol Prog Ser 85:55–68
Fodrie FJ, Rodriguez AB, Baillie CJ, Brodeur MC, Coleman SE, Gittman RK, Keller DA, Kenworthy MD, Poray AK, Ridge JT, Theuerkauf EJ, Lindquist NL (2014) Classic paradigms in a novel environment: inserting food web and productivity lessons from rocky shores and saltmarshes into biogenic reef restoration. J Applied Ecol 51:1314–1325
Geraldi NR, Powers SP, Heck KL, Cebrian J (2009) Can habitat restoration be redundant? Response of mobile fishes and crustaceans to oyster reef restoration in marsh tidal creeks. Mar Ecol Prog Ser 389:171–180
Giotta RE (1999) Distribution of American oysters (Crassostrea virginica) in South Carolina: interacting effects of predation, sedimentation, and water flow at varying tidal elevations. MS Thesis, University of Charleston
Gosselin LA, Qian PY (1997) Juvenile mortality in benthic marine invertebrates. Mar Ecol Prog Ser 146:265–282
Grabowski J, Kimbro D (2005) Predator-avoidance behavior extends trophic cascades to refuge habitats. Ecology 86:1312–1319
Grabowski JH, Powers SP (2004) Habitat complexity mitigates trophic transfer on oyster reefs. Mar Ecol Prog Ser 277:291–295
Grabowski JH, Hughes AR, Kimbro DL, Dolan MA (2005) How habitat setting influences restored oyster reef communities. Ecology 86:1926–1935
Grabowski JH, Hughes AR, Kimbro DL (2008) Habitat complexity influences cascading effects of multiple predators. Ecology 89:3413–3422
Gregalis KC, Johnson MW, Powers SP (2009) Restored oyster reef location and design affect responses of resident and transient fish, crab, and shellfish species in Mobile Bay, Alabama. Trans Amer Fish Soc 138:314–327
Hanke MH (2014) Landscape approaches to understanding the function of intertidal oyster reefs. PhD Dissertation. University of North Carolina Wilmington
Harwell HD, Posey MH, Alphin TD (2011) Landscape aspects of oyster reefs: effects of fragmentation on habitat utilization. J Exp Mar Biol Ecol 409:30–41
Hill J, Weissburg M (2013a) Habitat complexity and predator size mediate interactions between intraguild blue crab predators and mud crab prey in oyster reefs. Mar Ecol Prog Ser 488:209–219
Hill J, Weissburg M (2013b) Predator biomass determines the magnitude of non-consumptive effects (NCEs) in both laboratory and field environments. Oecologia 172:79–91
Hothorn T, Bretz F, Westfall P (2008) Simultaneous interference in general parametric models. Biometrical J 50:346–363
Hughes AR, Rooker K, Murdock M, Kimbro DL (2012) Predator cue and prey density interactively influence indirect effects on basal resources in intertidal oyster reefs. PLoS ONE 7:e44839
Hunt H, Scheibling R (1997) Role of early post-settlement mortality in recruitment of benthic marine invertebrates. Mar Ecol Prog Ser 155:269–301
Johnson KD, Smee DL (2014) Predators influence the tidal distribution of oysters (Crassostrea virginica). Mar Biol 161:1557–1564
Johnson KD, Grabowski JH, Smee DL (2014) Omnivory dampens trophic cascades in estuarine communities. Mar Ecol Prog Ser 507:197–206
Knights AM, Walters K (2010) Recruit–recruit interactions, density-dependent processes and population persistence in the eastern oyster Crassostrea virginica. Mar Ecol Prog Ser 404:79–90
Knights AM, Firth LB, Walters K (2012) Interactions between multiple recruitment drivers: post-settlement predation mortality and flow-mediated recruitment. PLoS ONE 7:e35096
Kulp RE, Peterson BJ (2013) Who controls whom? Linking the predator-prey dynamics between mud crabs and juvenile Eastern oysters to restoration efforts in the New York Metropolitan Region, Sect IV. In: Fernald SH, Yozzo DJ, Andreyko H (eds) Final reports of the tibor T. Polgar Fellowship Program, 2012. Hudson River Foundation, pp 1–32
Kulp R, Politano V, Lane H, Lombardi S, Paynter K (2011) Predation of juvenile Crassostrea virginica by two species of mud crabs found in the Chesapeake Bay. J Shellfish Res 30:1–6
Lin J (1990) Mud crab predation on ribbed mussels in salt marshes. Mar Biol 107:103–109
McDermott J (1960) The predation of oysters and barnacles by crabs of the family Xanthidae. Proc Penn Acad Sci 34:199–211
McDonald J (1982) Divergent life history patterns in the co-occurring intertidal crabs Panopeus herbstii and Eurypanopeus depressus (Crustacea: Brachyura: Xanthidae). Mar Ecol Prog Ser 8:173–180
Meyer D (1994) Habitat partitioning between xanthid crabs Panopeus herbstii and Eurypanopeus depressus on intertidal oyster reefs (Crassostrea virginica) in southeastern North Carolina. Estuaries 17:674–679
Micheli F, Peterson C (1999) Estuarine vegetated habitats as corridors for predator movements. Conserv Biol 13:869–881
Milke L, Kennedy V (2001) Mud crabs (Xanthidae) in Chesapeake Bay: claw characteristics and predation on epifaunal bivalves. Invert Biol 120:67–77
Nelson KA, Leonard LA, Posey MH, Alphin TD, Mallin MA (2004) Using transplanted oyster (Crassostrea virginica) beds to improve water quality in small tidal creeks: a pilot study. J Exp Mar Biol Ecol 298:347–368
Newell RIE, Alspach GS Jr, Kennedy VS, Jacobs D (2000) Mortality of newly metamorphosed eastern oysters (Crassostrea virginica) in mesohaline Chesapeake Bay. Mar Biol 136:665–676
Nowell ARM, Jumars PA (1984) Flow environments of aquatic benthos. Ann Rev Ecol Syst 15:303–328
O’Beirn FX, Heffernan PB, Walker RL (1996) Recruitment of the eastern oyster in coastal Georgia: patterns and recommendations. N Amer J Fisher Manag 16:413–426
O’Connor N, Grabowski J, Ladwig L, Bruno J (2008) Simulated predator extinctions: predator identity affects survival and recruitment of oysters. Ecology 89:428–438
Peterson CH (1979) Predation, competitive exclusion, and diversity in the soft-sediment benthic communities of estuaries and lagoons. In: Livingston RJ (ed) Ecological processes in coastal and marine systems. Plenum Press, New York, pp 233–264
Peterson CH (1982) The importance of predation and intra- and interspecific competition in the population biology of two infaunal suspension-feeding bivalves, Protothaca staminea and Chione undatella. Ecol Monog 52:437–475
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Rindone RR, Eggleston DB (2011) Predator-prey dynamics between recently established stone crabs (Menippe spp.) and oyster prey (Crassostrea virginica). J Exp Mar Biol Ecol 407:216–225
Rothschild B, Ault J, Goultetquer P, Heral M (1994) Decline of the Chesapeake Bay oyster population: a century of habitat destruction and overfishing. Mar Ecol Prog Ser 111:29–39
Seed R (1980) Predator-prey relationships between the mud crab Panopeus herbstii, the blue crab, Callinectes sapidus and the Atlantic ribbed mussel Guekensia (=Modiolus) demissa. Estuar Coast Mar Sci 11:445–458
Seed R (1993) Invertebrate predators and their role in structuring coastal and estuarine populations of filter feeding bivalves. In: Dame R (ed) Bivalve filter feeders. Springer, Berlin, pp 149–195
Seitz R, Lipcius R, Hines A, Eggleston D (2001) Density-dependent predation, habitat variation, and the persistence of marine bivalve prey. Ecology 82:2435–2451
Sih A, Englund G, Wooster D (1998) Emergent impacts of multiple predators on prey. Trends Ecol Evol 13:350–355
Soniat TM, Finelli CM, Ruiz JT (2004) Vertical structure and predator refuge mediate oyster reef development and community dynamics. J Exp Mar Biol Ecol 310:163–182
Toscano B, Griffen B (2012) Predatory crab size diversity and bivalve consumption in oyster reefs. Mar Ecol Prog Ser 445:65–74
Toscano B, Griffen B (2013) Predator size interacts with habitat structure to determine the allometric scaling of the functional response. Oikos 122:454–462
Woodin S (1981) Disturbance and community structure in a shallow water sand flat. Ecology 62:1052–1066
Acknowledgments
We would like to thank Dr. Ami Wilbur, Director of the UNCW Shellfish Hatchery, for supplying the oysters used in this study. We would also like to acknowledge Dr. Will White, Troy Alphin, and Marc Hanke at UNCW, Dr. Joel Fodrie of UNC-CH, and Rebecca Kulp of Stony Brook University for useful comments on this research. We would like to thank David Meyer of NOAA for the use of crab cages. Finally, symbols used for the conceptual diagrams were courtesy of the Integration and Application Network, University of Maryland Center for Environmental Studies (ian.umces.edu/symbols/).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Grassle.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Carroll, J.M., Marion, J.P. & Finelli, C.M. A field test of the effects of mesopredators and landscape setting on juvenile oyster, Crassostrea virginica, consumption on intertidal reefs. Mar Biol 162, 993–1003 (2015). https://doi.org/10.1007/s00227-015-2643-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-015-2643-7