Abstract
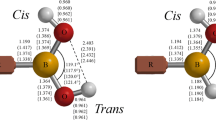
We have mapped the energy demands of the geometrical changes in donor–acceptor complexes BH3⋅NH3 and AlCl3⋅NH3 and in the course of their formation from their monomers. We have varied the individual geometrical parameters systematically and performed ab initio quantum chemical calculations for these structures. We investigated the energy requirements to change bond lengths and bond angles in both the monomers and complexes and the angles of torsion in the complexes. The changes of bond lengths require more energy in the monomers than in the complexes. The energies to change the acceptor bond angles in the monomers are markedly higher than in the complexes. The changes in the geometrical parameters during the complexation process are more moderate in donors than in acceptors, in agreement with prior experimental observations. The geometry versus energy variations related to the process of complexation are in agreement with the notion of relative rigidity of the donor parts and the more compliant nature of the acceptor parts as well as with the notion of competing effects in the structures of the complexes.
Similar content being viewed by others
REFERENCES
Hargittai, I. and Levy, J. B. Struct. Chem. 1999, 10, 387.
Horváth, V.; Kovács, A. and Hargittai, I. J. Phys. Chem. 2003, 107, 1197.
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Zakrzewski, V. G.; Montgomery, Jr., J. A.; Stratmann, R. E.; Burant, J. C.; Dapprich, S.; Millam, J. M.; Daniels, A. D.; Kudin, K. N.; Strain, M. C.; Farkas, O.; Tomasi, J.; Barone, V.; Cossi, M.; Cammi, R.; Mennucci, B.; Pomelli, C.; Adamo, C.; Clifford, S.; Ochterski, J.; Petersson, G. A.; Ayala, P. Y.; Cui, Q.; Morokuma, K.; Rabuck, A. D.; Raghavachari, K.; Foresman, J. B.; Cioslowski, J.; Ortiz, J. V.; Stefanov, B. B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Gomperts, R.; Martin, R. L.; Fox, D. J.; Keith, T.; Al-Laham, M. A.; Peng, C. Y.; Nanayakkara, A.; Gonzalez, C.; Challacombe, M.; Gill, P. M. W.; Johnson, B.; Chen, W.; Wong, M. W.; Andres, J. L.; Gonzalez, C.; Head-Gordon, M.; Replogle E. S. and Pople, J. A. GAUSSIAN98; Gaussian Inc.: Pittsburgh, Pennsywania, 1998.
Hargittai, M. and Hargittai, I. The Molecular Geometries of Coordination Compounds in the Vapour Phase; Akadémiai Kiadó: Budapest, 1977; pp. 68–71.
Hargittai, M. and Hargittai, I. J. Mol. Struct. 1977, 39, 79.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Horváth, V., Hargittai, I. Geometrical Changes and Their Energies in the Formation of Donor–Acceptor Complexes. Structural Chemistry 15, 233–236 (2004). https://doi.org/10.1023/B:STUC.0000021532.01536.21
Issue Date:
DOI: https://doi.org/10.1023/B:STUC.0000021532.01536.21