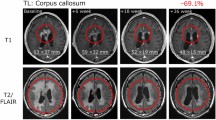
Abstract
To investigate the safety of combined Wilms tumor 1 peptide vaccination and temozolomide treatment of glioblastoma, a phase I clinical trial was designed. Seven patients with histological diagnosis of glioblastoma underwent concurrent radiotherapy and temozolomide therapy. Patients first received Wilms tumor 1 peptide vaccination 1 week after the end of combined concurrent radio/temozolomide therapy, and administration was continued once per week for 7 weeks. Temozolomide maintenance was started and performed for up to 24 cycles, and the observation period for safety encompassed 6 weeks from the first administration of maintenance temozolomide. All patients showed good tolerability during the observation period. Skin disorders, such as grade 1/2 injection-site reactions, were observed in all seven patients. Although grade 3 lymphocytopenia potentially due to concurrent radio/temozolomide therapy was observed in five patients (71.4 %), no other grade 3/4 hematological or neurological toxicities were observed. No autoimmune reactions were observed. All patients are still alive, and six are on Wilms tumor 1 peptide vaccination without progression, yielding a progression-free survival from histological diagnosis of 5.2–49.1 months. Wilms tumor 1 peptide vaccination was stopped in one patient after 12 injections by the patient’s request. The safety profile of the combined Wilms tumor 1 peptide vaccination and temozolomide therapy approach for treating glioblastoma was confirmed.



Similar content being viewed by others
Abbreviations
- CR:
-
Complete response
- CTCAE:
-
Common Terminology Criteria for Adverse Events
- DSMC:
-
Data Safety Monitoring Committee
- DTH:
-
Delayed-type hypersensitivity
- EORTC/NCIC:
-
European Organization for Research and Treatment of Cancer/National Cancer Institute of Canada
- FACS:
-
Fluorescence-activated cell sorting
- GBM:
-
Glioblastoma
- GTR:
-
Gross total resection
- mAbs:
-
Monoclonal antibodies
- NR:
-
No recurrence
- PR:
-
Partial resection
- Gy:
-
Gray
- IDH1:
-
Isocitrate dehydrogenase 1
- ORR:
-
Objective response rate
- OS:
-
Overall survival
- PBMCs:
-
Peripheral blood mononuclear cells
- PD:
-
Progressive disease
- PFS:
-
Progression-free survival
- PS:
-
Performance status
- RECIST:
-
Response Evaluation Criteria in Solid Tumors
- RT:
-
Radiotherapy
- RPA:
-
Recursive partitioning analysis
- TMZ:
-
Temozolmide
- TPS:
-
Total prognostic score
- WT1:
-
Wilms tumor 1
References
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJB et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466
Yu JS, Liu G, Ying H, Yong WH, Black KL et al (2004) Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific cytotoxic T-cells in patients with malignant glioma. Cancer Res 64:4973–4979
Yamanaka R, Homma J, Yajima N, Tsuchiya N, Sano M et al (2005) Clinical evaluation of personalized peptide vaccination for patients with recurrent glioma: results of a clinical phase I/II trial. Clin Cancer Res 11:4160–4167
Terasaki M, Shibui S, Narita Y, Fujimaki T, Aoki T et al (2010) Phase I trial of a personalized peptide vaccine for patients positive for human leukocyte antigen-A24 with recurrent or progressive glioblastoma multiforme. J Clin Oncol 29:337–344
Oka Y, Tsuboi A, Elisseeva OA, Udaka K, Sugiyama H (2002) WT1 as a novel target antigen for cancer immunotherapy. Curr Cancer Drug Targets 2:45–54
Tsuboi A, Oka Y, Ogawa H, Elisseeva OA, Tamaki H et al (1999) Constitutive expression of the Wilms’ tumor gene WT1 inhibits the differentiation of myeloid progenitor cells but promotes the proliferation in response to granulocyte-colony stimulation factor (G-CSF). Leuk Res 23:499–505
Oka Y, Udaka K, Tsuboi A, Elisseeva OA, Ogawa H et al (2000) Cancer immunotherapy targeting Wilms’ tumor gene WT1 product. J Immunol 164:1873–1880
Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM et al (2009) The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res 15:5323–5337
Oji Y, Suzuki T, Nakano Y, Maruno M, Nakatsuka S et al (2004) Overexpression of the Wilms’ tumor gene WT1 in primary astrocytic tumors. Cancer Sci 95:822–827
Izumoto S, Tsuboi A, Oka Y, Suzuki T, Hashiba T et al (2008) Phase II clinical trial of Wilms tumor 1 peptide vaccination for patients with recurrent glioblastoma multiforme. J Neurosurg 108:963–971
Khan RB, Raizer JJ, Malkin MG, Bazylewicz KA, Abrey LE (2002) A phase II study of extended low-dose temozolomide in recurrent malignant gliomas. Neuro Oncol 4:39–43
Brandes AA, Tosoni A, Cavallo G, Bertorelle R, Gioia V et al (2006) Temozolomide 3 weeks on and 1 week off as first-line therapy for recurrent glioblastoma: phase II study from Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO). Br J Cancer 95:1155–1160
Wick A, Felsberg J, Steinbach JP, Herrlinger U, Platten M et al (2007) Efficacy and tolerability of temozolomide in an alternating weekly regimen in patients with recurrent glioma. J Clin Oncol 25:3357–3361
Perry JR, Belanger K, Mason WP, Fulton D, Kavan P et al (2010) Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J Clin Oncol 28:2051–2057
Kong DS, Lee JI, Kim JH, Kim ST, Kim WS et al (2010) Phase II trial of low-dose continuous (metronomic) treatment of temozolomide for recurrent glioblastoma. Neuro Oncol 12:289–296
Abacioglu U, Caglar HB, Yumuk PF, Akgun Z, Atasoy BM et al (2011) Efficacy of protracted dose-dense temozolomide in patients with recurrent high-grade glioma. J Neuro Oncol 103:585–593
Omuro A, Chan TA, Abrey LE, Khasraw M, Reiner AS et al (2013) Phase II trial of continuous low-dose temozolomide for patients with recurrent malignant glioma. Neuro Oncol 15:242–250
Chiba Y, Hashimoto N, Tsuboi A, Oka Y, Murao A et al (2010) Effects of concomitant temozolomide and radiation therapies on WT1-specific T cells in malignant glioma. Jpn J Clin Oncol 40:395–408
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Momota S, Narita Y, Miyakita Y, Shibui S (2013) Secondary hematological malignancies associated with temozolomide in patients with glioma. Neuro Oncol 15:1445–1450
Oka Y, Tsuboi A, Taguchi T, Osaki T, Kyo T et al (2004) Induction of WT1 (Wilms’ tumor gene)-specific cytotoxic T lymphocytes by WT1 peptide vaccine and the resultant cancer regression. Proc Natl Acad Sci U S A 101:13885–13890
Tsuboi A, Oka Y, Udaka K, Murakami M, Masuda T et al (2002) Enhanced induction of human WT1-specific cytotoxic T lymphocytes with a 9-mer WT1 peptide modified at HLA-A*2402-binding residues. Cancer Immunol Immunother 51:614–620
Gonzalez-Galarza FF, Christmas S, Middleton D, Jones AR (2011) Allele frequency net: a database and online repository for immune gene frequencies in worldwide populations. Nucleic Acid Res 39:D913–D919
Gorlia T, van den Bent MJ, Hegi ME, Mirimanoff RO, Weller M et al (2008) Nomograms for predicting survival of patients with newly diagnosed glioblastoma: prognostic factor analysis of EORTC and NCIC trial 26981-22981/CE.3. Lancet Oncol 9:29–38
Li J, Wang M, Won M, Shaw EG, Coughlin C et al (2011) Validation and simplification of the Radiation Therapy Oncology Group recursive partitioning analysis classification for glioblastoma. Int J Radiat Oncol Biol Phys 81:623–630
Capper D, Zentgraf H, Balss J, Hartmann C, von Deimling A (2009) Monoclonal antibody specific for IDH1 R132H mutation. Acta Neuropathol 118:599–601
Stupp R, Dietrich PY, Ostermann Kraljevic S, Pica A, Maillard I, Maeder P et al (2002) Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide. J Clin Oncol 20:1375–1382
Su YB, Sohn S, Krown SE, Livingston PO, Wolchok JD et al (2004) Selective CD4_ lymphopenia in melanoma patients treated with temozolomide: a toxicity with therapeutic implications. J Clin Oncol 22:610–616
Fujimoto Y, Hashimoto N, Kinoshita M, Miyazaki Y, Tanaka S et al (2012) Hepatitis B virus reactivation associated with temozolomide for malignant glioma: a case report and recommendation for prophylaxis. Int J Clin Oncol 17:290–293
Appay V, Voelter V, Rufer N, Reynard S, Jandus C et al (2007) Combination of transient lymphodepletion with busulfan and fludarabine and peptide vaccination in a phase I clinical trial for patients with advanced melanoma. J Immunother 30:240–250
Heimberger AB, Sun W, Hussain SF, Dey M, Crutcher L et al (2008) Immunological responses in a patient with glioblastoma multiforme treated with sequential courses of temozolomide and immunotherapy: case study. Neuro Oncol 10:98–103
Park SD, Kim CH, Kim CK, Park JA, Sohn HJ et al (2007) Cross-priming by temozolomide enhances antitumor immunity of dendritic cell vaccination in murine brain tumor model. Vaccine 25:3485–3491
Kim CH, Woo SJ, Park JS, Kim HS, Park MY et al (2007) Enhanced antitumour immunity by combined use of temozolomide and TAT-survivin pulsed dendritic cells in a murine glioma. Immunology 122:615–622
Jordan JT, Sun W, Farzana Hussain S, DeAngulo G, Prabhu SS et al (2008) Preferential migration of regulatory T cells mediated by glioma secreted chemokines can be blocked with chemotherapy. Cancer Immunol Immunother 57:123–131
Banissi C, Ghiringhelli F, Chen L, Carpentier AF (2009) Treg depletion with a low-dose metronomic temozolomide regimen in rat glioma model. Cancer Immunol Immunother 58:1627–1634
Sanchez-Perez LA, Choi BD, Archer GE, Cui X, Flores C et al (2013) Myeloablative temozolomide enhances CD8 T-cell responses to vaccine and is required for efficacy against brain tumors in mice. PLoS One 8:e59082
North RJ (1982) Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med 155:1063–1074
Dudley ME, Robbins PF, Yang JC, Yang JC, Hwu P et al (2002) Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 298:850–854
Sampson JH, Aldape KD, Archer GE, Coan A, Desjardins A et al (2011) Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro Oncol 13:324–333
Fadul CE, Fisher JL, Gui J, Hampton TH, Côté AL et al (2011) Immune modulation effects of concomitant temozolomide and radiation therapy on peripheral blood mononuclear cells in patients with glioblastoma multiforme. Neuro Oncol 13:393–400
Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH et al (2010) Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol 28:4722–4729
Acknowledgments
The authors would like to thank Ms. Tomoe Umeda, Department of Cancer Immunotherapy, for her technical assistance. They would also thank Ms. Mariko Kakinoki and Ms. Yuko Komiyama, Department of Neurosurgery, for their secretarial assistance. This work was supported in part by Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (No. 23592123 to Naoya Hashimoto and No. 22591609 to Akihiro Tsuboi).
Conflict of interest
The funding source has no involvement in the study design, the collection, analysis, and interpretation of data, and in the writing of the report.
Ethical standards
This study was conducted according to the principles expressed in the Declaration of Helsinki and approved by the ethics review boards of the Osaka University Faculty of Medicine. Written informed consents were obtained from all patients enrolled.
Author information
Authors and Affiliations
Corresponding author
Additional information
Naoya Hashimoto and Akihiro Tsuboi have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hashimoto, N., Tsuboi, A., Kagawa, N. et al. Wilms tumor 1 peptide vaccination combined with temozolomide against newly diagnosed glioblastoma: safety and impact on immunological response. Cancer Immunol Immunother 64, 707–716 (2015). https://doi.org/10.1007/s00262-015-1674-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-015-1674-8